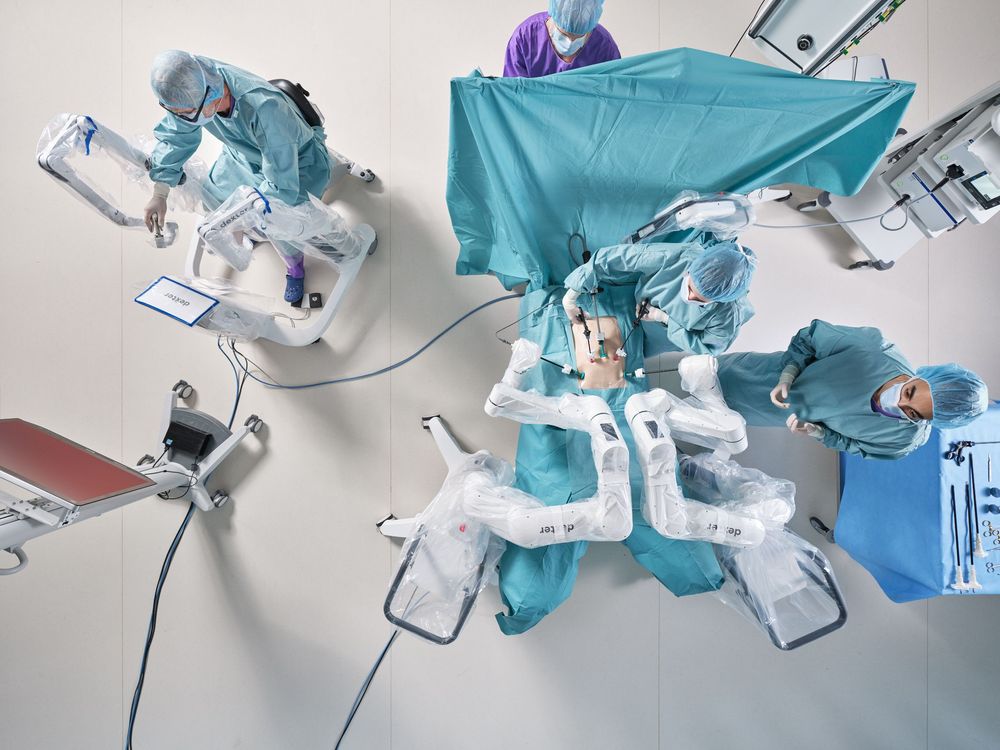
With the receipt of the De Novo Marketing Authorization from the US Food and Drug Administration Distalmotion is set to bring its surgical robot, Dexter into the US. This will expand access to its robotic assisted surgery in the hospital outpatient and ambulatory surgery centers, where over 90 percent of inguinal hernia repairs are currently performed.
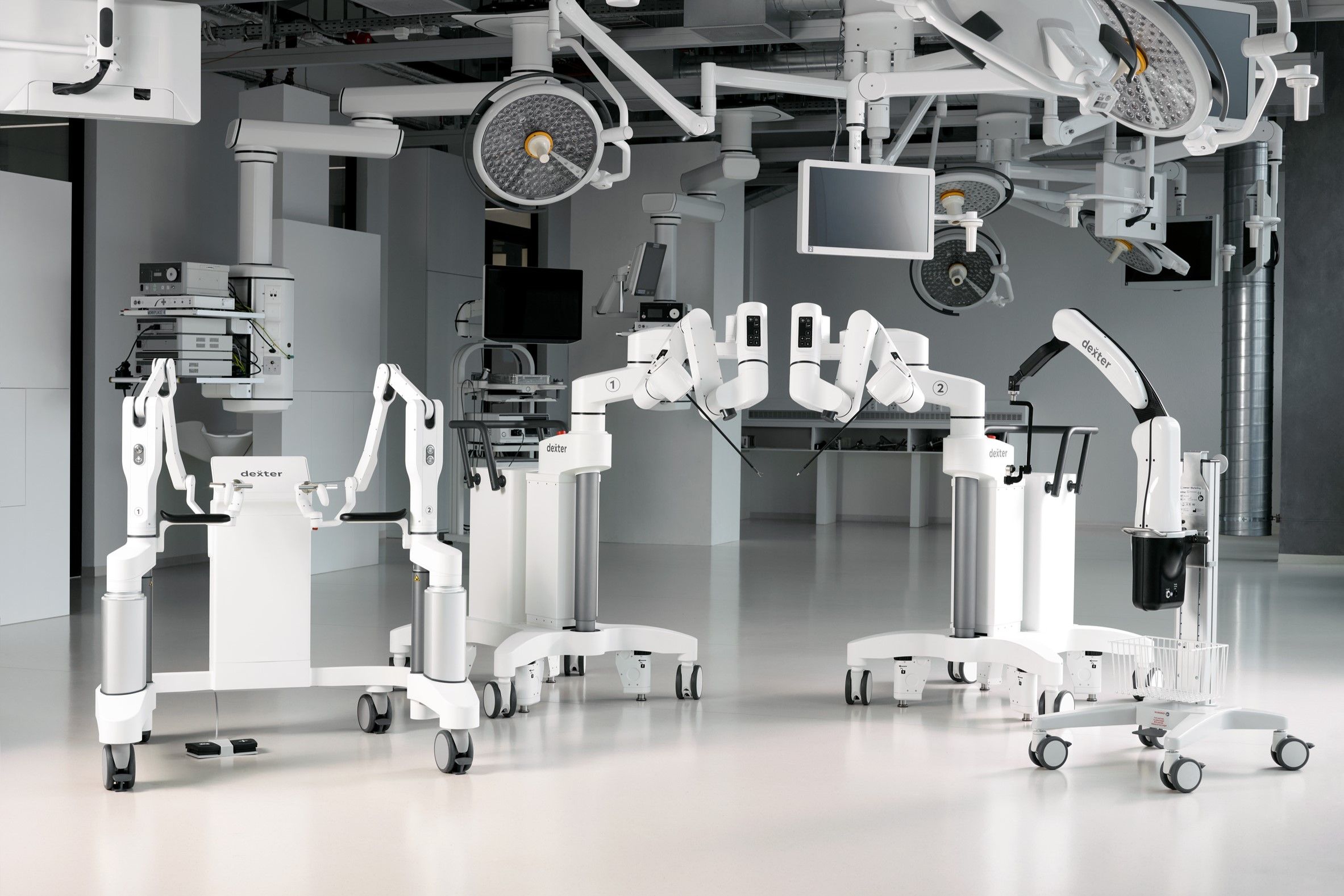
Based in Lausanne, Distalmotion is focused on developing a robotic platform for high-volume procedures and bringing the benefits of robotic-assisted surgery to outpatient settings without overnight stay at the hospital. Dexter’s mobile design, combined with its small form factor, make it particularly well-suited to these environments, allowing hospitals and ambulatory surgery centers (ASCs) to optimize resources while still accessing the latest technologies and best-in-class surgical tools.
Receiving the De Novo approval by the U.S. Food and Drug Administration (FDA) allows Distalmotion to market the Dexter Surgical Robot for adult inguinal hernia repair. 90% of inguinal hernia repairs in the United States are currently performed in the hospital outpatient and ambulatory surgery centers.
With more than 1,300 patients successfully treated in Europe, Distalmotion’s extensive experience has paved the way for bringing this innovation to the U.S. market. The De Novo pathway, introduced by the FDA in 1997, allows companies to receive FDA clearance for low or medium risk medical devices with no existing predicate, or no substantial equivalence, ensuring safety and effectiveness through risk-based evaluation. It uses general or special controls when general controls alone are insufficient.
“We’re excited to bring Dexter to the U.S. market and empower healthcare facilities with a robotic solution that addresses the barriers of cost, space, and workflow disruption,” commented Greg Roche, Distalmotion’s CEO. “Our goal is to enhance existing practices with robotics that support—not disrupt—the way surgical teams operate.”
Dexter seamlessly integrates into existing operating workflows and is fully compatible with current operating room equipment, protecting hospitals’ existing investments. Additionally, its single-use instruments remove the complexities of reprocessing, further enhancing workflow efficiency. Dexter’s design enables direct and quick access to the patient from the sterile surgeon console, allowing surgeons the flexibility to choose the best technique for each step of the procedure. Dexter is designed to be the “surgeon’s robot” that provides the physician complete control of the procedure to optimize patient outcomes.
“Inguinal hernia repair is an excellent first indication for Dexter. Access to robots for these procedures has historically been a challenge. Dexter’s design will undoubtedly enable more patients to benefit from robotic assisted surgery,” said William Hope, MD, Associate Professor of Surgery, UNC Chapel Hill, General Surgery Residency Program Director, New Hanover Regional Medical Center (Novant Health).
Ryan Broderick, MD, Associate Professor of Surgery, UCSD shared, “Dexter delivers exceptional performance and without limitations. Its open platform allows flexibility, whether in terms of the surgeon console or the option to choose your preferred vision system, stapling, and advanced energy. We are seeing the continued shift of procedures moving to the outpatient setting. The footprint and cost, both less than other solutions, enable Dexter to be the ideal robot for outpatient sites of care and those patients. Dexter is the solution for everyday surgeries, and that’s what the market needs.”